Table of Contents
Environmental Studies is a captivating field that encompasses a range of disciplines, including chemistry, physical science, life science, agriculture, health, and sanitary engineering. Its significance is evident in CTET and State TET Exams, where it features around 30 questions, making it a subject of interest for aspiring candidates.
To excel in the CTET Exam, studying the NCERT books from class 1 to 8 is highly recommended, as they lay a strong foundation for understanding Environmental Studies. This article aims to introduce some key points related to this fascinating subject and present intriguing facts that shed light on the intricate connections within our environment.
THINGS WE KNOW
1. Matter:
Anything that has mass and occupies space is called matter. There are three kinds of matter i.e. solid, liquid and gas on the basis of physical character and on the basis of chemical properties it is classified into element, compound and mixture.
Classification of Matter on Basis of Physical Character:
- Solid: Matter which has a fixed shape and volume. e.g. bricks, table, chair, book etc.
- Liquid: Matter which have a fixed volume but not a definite shape. Liquid takes the shape of vessels in which it is kept or stored. e.g. milk, water, oil, fuel etc.
- Gas: Substance which has neither fixed shape nor volume. These substances are easily affected by the pressure. e.g. air, hydrogen gas, LPG, CNG.
Classification of Matter on Basis of Chemical Character:
Element: The matter which is made up of the same type of atoms is called an element. The element may be metal or non-metal. Metals are found in the solid state except mercury which is in liquid state. Metals have characteristics of malleability (which can be drawn into sheets) and ductility (which can be drawn into wire).
Metals are tough, have the property to shine, and usually conduct electricity and heat, the density of metals are higher than other substance. e.g. iron, copper, zinc etc. while non-metal does not have properties of malleability, ductility, shine and density like metals and they have also lower melting and boiling points in comparison to metals e.g. silicon, sulphur, carbon etc.
Compounds: They are made by a combination of two or more elements in a definite proportion. Compounds have fixed chemical formulae and their properties is different from the element from which it is made. e.g. Water (H₂O), Carbon Dioxide (CO₂) Calcium Carbonate/Chalk (CaCO₃) and Lime (CaO) etc.
Check CTET 2020 Exam Pattern And Syllabus For Paper 1
Mixture: They are also made by a combination of two or more elements and compounds but these elements and compounds are not present in a definite ratio and proportion. Mixtures can be separated by process of separation either chemical or physical, e.g. air, soil etc.
Alloy: It is a mixture of metal or a mixture of metal with another element. Alloys may be a solid solution of metal or a mixture of metallic phases. Inter-metallic compounds are alloys with a defined stoichiometry and crystal structure. The properties of alloys are different from its constituent elements. e.g. bronze, steel, brass etc.
|
Alloy and Its Constituent Elements |
|
|
Alloys |
Constituent Elements |
| Steel | Iron and Carbon |
| Stainless steel | Iron, Carbon, Nickel and Chromium |
| Bronze | Copper and Tin |
| Brass | Copper and Zinc |
| Duralumin | Aluminum, Bronze, Manganese and Magnesium |
| 22 carat Gold | Gold, Copper |
Note: Bronze It is the first alloy to be used. It has been used since very ancient times. It is a strong alloy and is used to make cannons, guns, statues and vessels.
2. Separation Techniques:
There are various separation techniques used for separating different mixtures.
A. Separation of Solid Components:
i. Manual Separation: Separation done by hand. e.g. separating concrete from rice, pulse etc.
ii. Threshing: It is the process of separating the edible part or grain from the stalk and husk, that surrounds the grain. Threshing is done during grain preparation after harvesting. For threshing, these days threshing machines are being used.
iii. Winnowing: in this process, a current of air is blown through the grain to separate the chaff surrounding the grain. It is done after the threshing.
iv. Filtration: This process helps in filtering the fine matter present along with the grain. In this process, a fine net is used. e.g, separation of sand and concrete, filtering the flour.
v. Magnetic Separation: In this technique, the magnet is used. This can be used to separate iron, cobalt, nickel, steel etc.
B. Separation of Solid and Liquid:
Sedimentation: In this process, liquid containing suspended solid particles is left for a while in a container, and slowly solid particles start to settle down in the bottom of the container. This settling down of solid particles is caused by the effect of gravity.
Decantation: In this technique, the liquid is transferred from one vessel to another without moving or disturbing the liquid or mixture. Before this process sedimentation is done and after settling down of solid or heavier liquid, surface liquid material which is floating on the upper surface is transferred.
Filtration: In this technique of separation net or filter paper/membrane is used. A mixture containing solid and liquid is passed through net/membrane/filter paper and in the process, solid particles are trapped in the net or membrane while liquid passes it easily.
Evaporation: In this process of separation, a mixture containing solid and liquid is heated so that the liquid evaporates and the solid is left behind as residue. e.g. separation of sugar and salt from solution.
Centrifugation: In this technique of separation, the mixture is rotated and due to force of rotation and gravity, solid particles settle down at the bottom rapidly in this way liquid and solid can be separated e.g. cream from milk, butter from milk etc.
Distillation: In this technique of separation mixture containing solid and liquid is heated and vapor formed during heating is collected and condensed in a specific container in this way desired component is separated.
C. Separation of Liquid from Liquid Mixture:
Separation Cone: It is used to separate two immiscible liquid or liquid which does not mix. When two immiscible liquids are present in the cone the liquid will form a bi-layer. One liquid will be present in the upper layer while the other is present in the bottom layer, through this cone bottom layer liquid can be easily drained. So only the upper layer of liquid will be left in a cone and in this way liquids are separated.
Fractional Distillation: In this process, liquid is heated at different temperatures and vapour is collected and condensed in separate containers for each temperature, Crude petroleum is heated at different temperatures to get Petrol, diesel, kerosene etc. So, the process of fractional distillation is used in separating petroleum products.
CTET EVS Study Notes PDF
Candidates preparing for the CTET Exam must check out the CTET EVS Study Notes PDF Link for easy access to study material on the Things We Know under the CTET EVS Section.
Click Here to Access CTET EVS Study Notes PDF on Things We Know



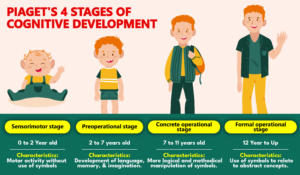 Piaget's 4 Stages of Cognitive Developme...
Piaget's 4 Stages of Cognitive Developme...
 स्वर व व्यंजन - �...
स्वर व व्यंजन - �...
 500+ पर्यायवाची श�...
500+ पर्यायवाची श�...








